CRP (C-reactive protein)
The biomarker of choice to aid the diagnosis of infection and inflammatory disorders
-
 Fast results
Fast results
-
 Early detection of inflammation
Early detection of inflammation
-
 Evaluation of infection
Evaluation of infection
CRP testing in the emergency department
When a patient is admitted with signs and symptoms of an infection, a C-reactive protein (CRP) test is used as an aid in the detection and evaluation of infection, tissue injury, inflammatory disorders, and associated diseases. CRP is valuable as an inflammation marker at the point-of-care, primary care and lab settings.
Increased CRP levels could be associated with bacterial, viral and fungal infections, sepsis, various forms of arthritis and several other infectious and inflammatory conditions [1]. CRP is an acute-phase protein produced by the liver and released into the blood a few hours after the onset of an infection, inflammation or tissue injury.
Because of the non-specific nature of the acute-phase response which leads to increased CRP levels, CRP should be used as a diagnostic aid in the clinical assessment of a patient [2].
Immunoassay products and solutions

CRP values for evaluation of infection
When faced with a patient with signs and symptoms of an infection, the physician faces the clinical dilemma of:
- Administering antibiotics in a timely manner [3].
- Avoiding inappropriate usage of antibiotics. Misuse of antibiotics
when not required has contributed to the problem of
multiresistant bacterial infections, which are very difficult to
treat [4].
Interpreting the CRP value
- Clinical cut-offs can be used to determine if the infection is bacterial or viral [5].
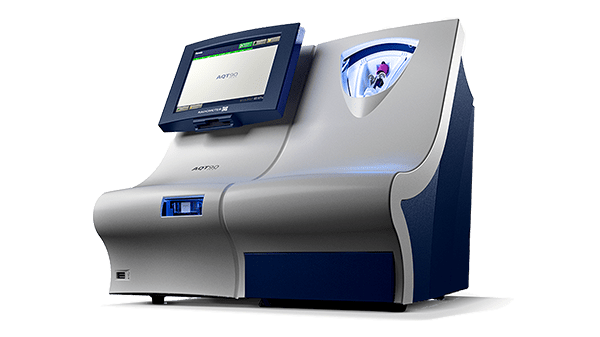
CRP test on the AQT90 FLEX analyser
The AQT90 FLEX immunoassay analyser is a benchtop analyser that brings rapid biomarker assessment capabilities right to the patient’s bedside.
The AQT90 FLEX analyser delivers quantitative results on the CRP assay in less than 13 minutes, accelerating infection and inflammatory disorder diagnosis in the ED to support rapid clinical decision making.
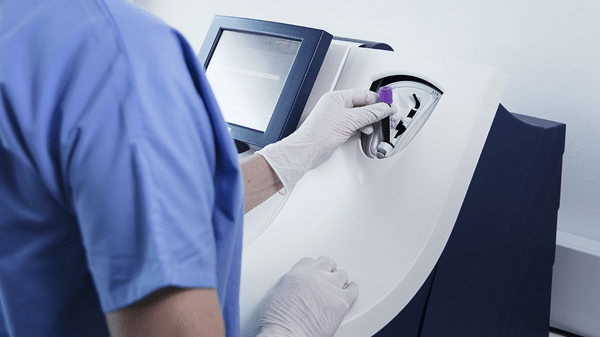
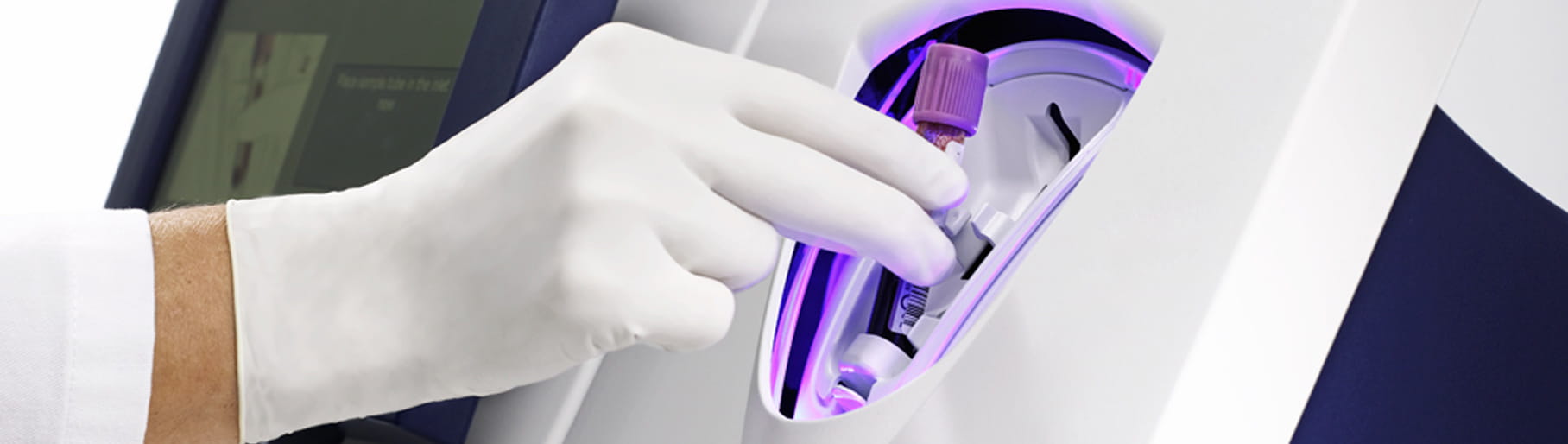
The AQT90 FLEX CRP assay can be used both in the lab and at the point of care without any sample or assay preparations, providing a fast result without any exposure to blood. Its closed tube system makes CRP testing easy: the operator simply inserts the sample tube into the tube holder in the sample port and the analyser performs all assay steps automatically.
All necessary reagents are included in the test cartridges, which remain stable onboard the analyser for 20 days ensuring maximum availability and uptime.
Key benefits of CRP on the AQT90 FLEX analyser
- No blood exposure: Closed system
- No sample or assay preparation
- Sample types: EDTA and lithium-heparin
- Specimen types: Venous whole blood and plasma
- Sample tubes: Fits most 13 × 75 mm standard tubes
- No presence of hook effect, carry-over or known interferences
CRP assay specifics
References
1. Testing. com. C-Reactive Protein (CRP) Test. https://www.testing.com/tests/c-reactive-protein-crp/. Accessed July 2022.
2. Seeger C et al. Acute care testing handbook 2014.
3. Joo YM et al. Impact of timely antibiotic administration on outcomes in patients with severe sepsis and septic shock in the emergency department. Clinical and Experimental Emergency Medicine 2014;1, 1.
4. Lee Ventola C. The Antibiotic Resistance Crisis
Part 1: Causes and Threats. P&T 2015; 40, 4.
5. Review Criteria for Assessment of C Reactive Protein (CRP), High Sensitivity C-Reactive Protein (hsCRP) and Cardiac C-Reactive Protein (cCRP) Assays - Guidance for Industry and FDA Staff. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/review-criteria-assessment-c-reactive-protein-crp-high-sensitivity-c-reactive-protein-hscrp-and. Assessed Dec 2020.
Cookies are used on this website
Use of cookiesPlease enter a valid email
We will be sending an e-mail invitation to you shortly to sign in using Microsoft Azure AD.
It seems that your e-mail is not registered with us
Please click "Get started" in the e-mail to complete the registration process
Radiometer is using Microsoft AZURE Active Directory to authenticate users
Radiometer uses Azure AD to provide our customers and partners secure access to documents, resources, and other services on our customer portal.
If your organization is already using Azure AD you can use the same credentials to access Radiometer's customer portal.
Key benefits
- Allow the use of existing Active Directory credentials
- Single-sign on experience
- Use same credentials to access future services
Request access
You will receive an invitation to access our services via e-mail when your request has been approved.
When you accept the invitation, and your organization is already using AZURE AD, you can use the same credentials to access Radiometer's customer portal. Otherwise, a one-time password will be sent via e-mail to sign in.