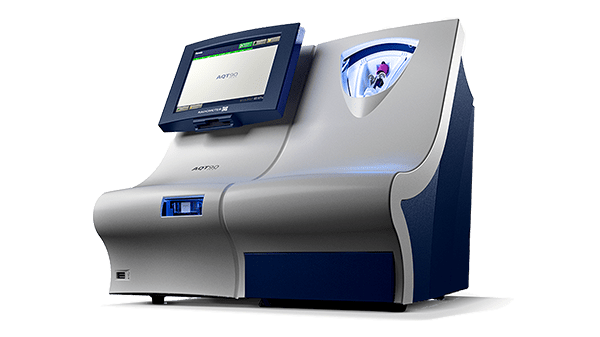
AQT90 FLEX immunoassay analyser
Point-of-care biomarker testing made simple
Check with your local representative for availability in specific markets
-
 Accelerate patient flow
Accelerate patient flow
-
 Make critical decisions fast
Make critical decisions fast
-
 Ensure reliable results
Ensure reliable results
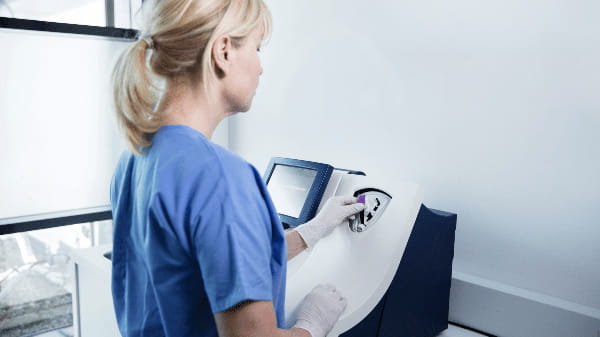
Accelerate patient flow with point-of-care testing
Point-of-care testing (POCT) provides a significant reduction in turnaround times [1], has the potential to improve patient care, workflow efficiency [2], and mitigate overcrowding in the emergency department [3].
With the AQT90 FLEX analyser’s closed tube system, ease of use at point of care is taken to the next level.
Automatic pipetting and automatic patient ID look-up mean minimal handling—simply Drop‘n’Go.
- Simply insert the collection tube into the AQT90 FLEX analyser
- Get your test results delivered directly through the HIS/LIS system to the medical records
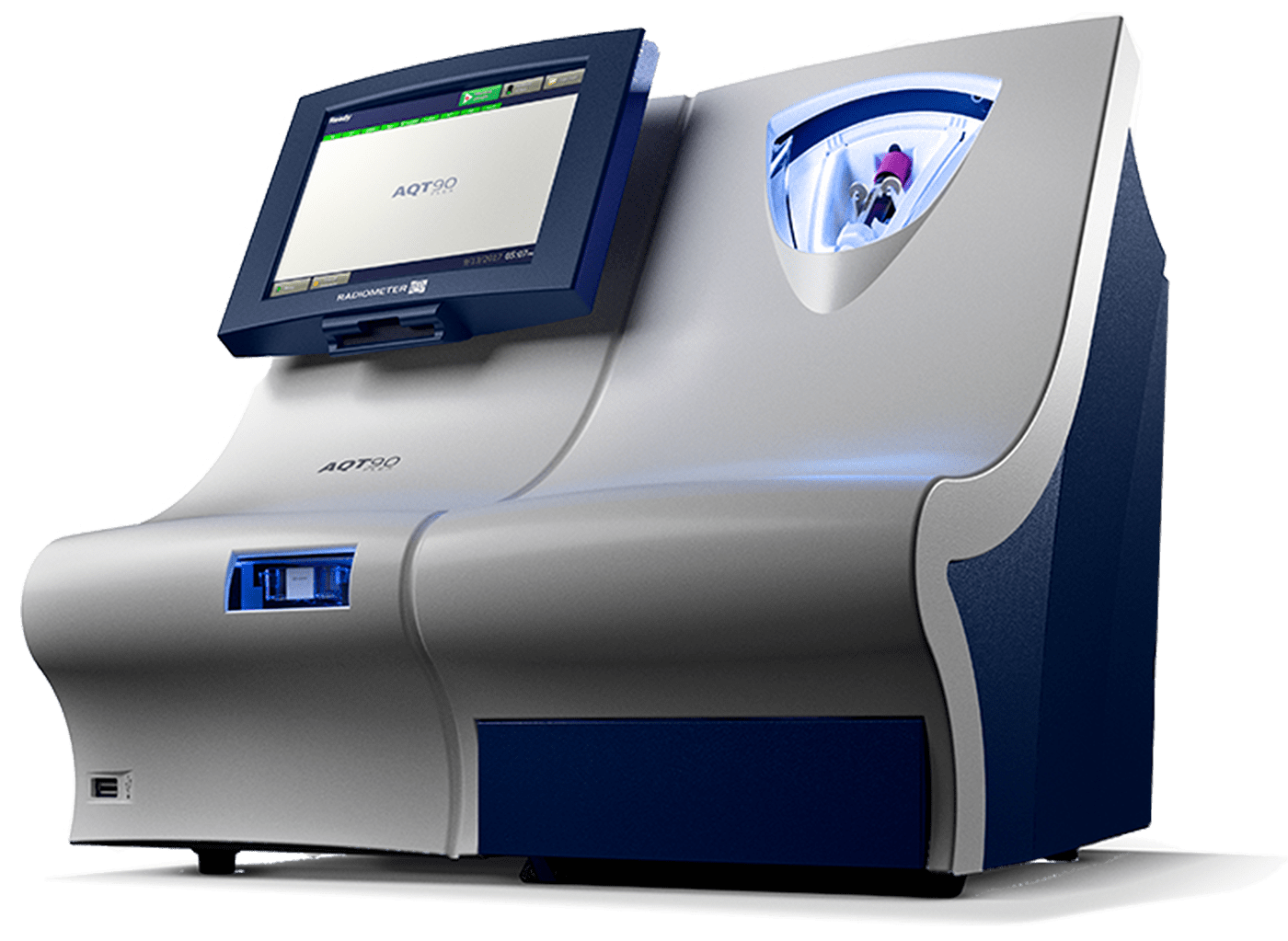
5. Test cartridges inlet
- Up to 240 tests when fully loaded
- 24 hrs. onboard stability for CAL cartridge
- Inventory alert when test number is low
3. Drop ’n’ Go
- Directly insert the closed sample tube
- Automatic pipetting
- Automatic mixing
- Test on both whole blood and plasma specimens*
- Multiple tube types: EDTA, Heparin & Citrate**
- Multiple tests can be run simultaneously.
*For the D-dimer assay, only whole blood samples can be used
**Citrate only applies for the D-dimer test
6. Connectivity
- HIS/LIS connectivity including automatic ‘patient ID lookup’
- Complete data capture through continous synchronization and data sent through HIS/LIS to the medical records
- Monitor analyzers, manage operators and quality controls across multiple sites
7. Barcode Reader
- No manual data entry needed
- Automatic reading of test cartridges
1. Customizable user interface
- Adjustable touch screen
- Onboard tutorials
- Customizable test selection
- Traffic light indicating current status
2. Quality Control (QC) Systems
Build in QC system automatical performs:
- Environmental checks
- Process checks
- Preanalytical checks
Ready-to-use liquid quality control (LQC) tubes
4. Solution Pack ‘Closed System’
- One solution pack for all tests
- Collection of waste
- Can be stored at room temperature
- Monitors the number of tests used
- Up to 200 tests before replacement is needed
- Disposal of the whole pack to avoid contact with blood
Testing in the Simple Zone with the AQT90 FLEX
![]()
![]()
![]()
![]()
![]()
Accelerate patient flow with the AQT90 FLEX analyzer
Click here to renew consent
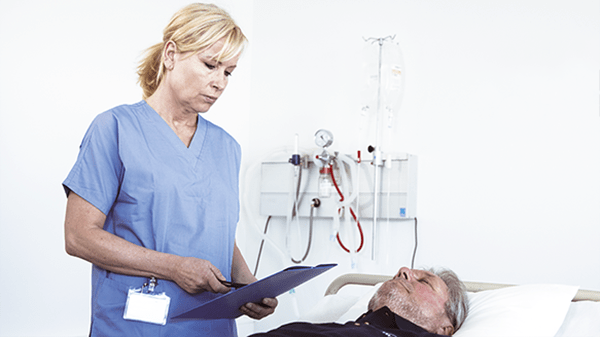

Make critical decisions fast
The AQT90 FLEX analyser delivers reliable results with a fast turnaround time. It has the capability to run up to 5 different tests per blood sample. Supporting you in making timely diagnosis of - or ruling out - several acute conditions.
You get results in 11-21 minutes based on the biomarker you’re testing. The immunoassay analyser allows you to initiate up to 30 tests per hour—you can manage more patients at the same time.
The AQT90 FLEX analyser covers an extensive range of critical biomarkers at POC.
Read more about the extensive range of biomarkers which aid in the diagnosis of:
Procalcitonin (PCT) assay aids in the diagnosis of sepsis, a serious and potentially fatal condition.
CRP test – lab quality at the POC
C-reactive protein (CRP) is an infection marker used as an aid in the detection of infection and inflammatory disorders in both point-of-care and lab settings.


Ensure reliable results
Make decisions fast without the worry of biotin interference
The AQT90 FLEX immunoassay analyser and assays have been tested for biotin interference. The results show no notable biotin interference in concentrations up to 2.6 mg/L (2,600 ng/mL), which is more than twice the amount recommended by the FDA [4].
Meet your regulatory needs
Meet compliance and regulatory demands with the AQT90 FLEX immunoassay analyser. The analyser includes:
- 24/7 connectivity to HIS/LIS directly
- Reliable results with built-in QC
You can also manage multiple AQT90 FLEX analysers with the AQURE POC IT solution. AQURE allows you to monitor analysers and manage operators and quality controls across multiple sites from one central location. With AQURE POC IT solution, you can stay ahead of compliance and accreditation needs without leaving your desk.


References
1. Nørgaard B, Mogensen CB. Blood sample tube transportation system versus point of care technology in an ED; effect on time from collection to reporting? A randomized trial. SJTREM 2012; 20, 71.
2. Larsson A et al. The state of point of care testing: a European perspective. Ups J Med Sci 2015; 120,1: 1-10.
3. St John A & Price C. Benefits of point of care testing in the emergency department. acutecaretesting.org 2018
4. FDA Safety Communication on Biotin. Nov 2017 ~ Nov 2019
Cookies are used on this website
Use of cookiesPlease enter a valid email
We will be sending an e-mail invitation to you shortly to sign in using Microsoft Azure AD.
It seems that your e-mail is not registered with us
Please click "Get started" in the e-mail to complete the registration process
Radiometer is using Microsoft AZURE Active Directory to authenticate users
Radiometer uses Azure AD to provide our customers and partners secure access to documents, resources, and other services on our customer portal.
If your organization is already using Azure AD you can use the same credentials to access Radiometer's customer portal.
Key benefits
- Allow the use of existing Active Directory credentials
- Single-sign on experience
- Use same credentials to access future services
Request access
You will receive an invitation to access our services via e-mail when your request has been approved.
When you accept the invitation, and your organization is already using AZURE AD, you can use the same credentials to access Radiometer's customer portal. Otherwise, a one-time password will be sent via e-mail to sign in.
